Northwire Contributes to the USP Class VI National Discussion Surrounding Medical Equipment Manufacturing
Published by Northwire Inc. on May 05, 2015
Design News Direct and Packaging Digest Direct are part of the Informa Markets Division of Informa PLC
Informa PLC
ABOUT US
INVESTOR RELATIONS
TALENT
This site is operated by a business or businesses owned by Informa PLC and all copyright resides with them. Informa PLC's registered office is 5 Howick Place, London SW1P 1WG. Registered in England and Wales. Number 8860726.

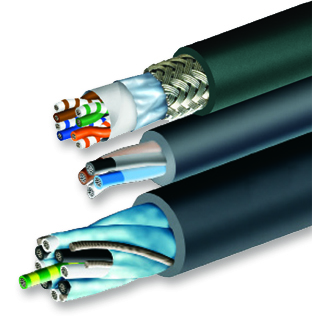
Northwire, as a recognized international design and manufacturer of technical wire and cable, custom cable, retractable cords, cable assemblies, connectors and harnesses, the contract manufacturing company shares key insights into the meticulous world of medical device testing. With a full range ...
Northwire, as a recognized international design and manufacturer of technical wire and cable, custom cable, retractable cords, cable assemblies, connectors and harnesses, the contract manufacturing company shares key insights into the meticulous world of medical device testing. With a full range of cutting-edge testing capabilities and extensive industry and agency certifications, Northwire is well-equipped to handle requests for medical wire requiring FDA approvals, RoHS2 and REACH compliant, or food grade materials. In addition to conflict free mineral adherence, the cable manufacturer offers USP Class VI tested materials.
Published by Northwire Inc. on May 05, 2015

T: 800-468-1516
F: 715-294-3727
Address
110 Prospect Way
Osceola, WI
54020
United States
View map
T: 800-468-1516
F: 715-294-3727
Address
110 Prospect Way
Osceola, WI
54020
United States
View map
